Abbott plans to ship 20 million antibody tests in the US. And European regulators for an antibody test on another of its platforms.
Abbott Launches Covid 19 Antibody Test Abbott Newsroom
The COVID-19 antibody test we use at Nuffield Health is very accurate and its been approved by Public Health England.
Best antibody test for covid 19 abbott. NAATs have detected SARS-CoV-2 RNA in some peoples respiratory specimens long after they have recovered from COVID-19 3 months. There is an overwhelming demand for an antibody test for covid-19 and companies globally are racing to produce them. Abbott Covid Antibody Test Methodology And Save Your money.
In June and beyond as we expand our testing capabilities to our Alinity i lab system. Abbott Labs Ships Over 1 Million Rapid Coronavirus Tests To Every State. Has launched a new antibody test to identify people who have been infected with COVID-19.
Abbotts antibody assay was tested on 1200 specimens whereas Epitopes tests were run on 54 samples from healthy people and just 20 and 30 cases. For persons with COVID-19 testing is not recommended to determine when infection has resolved when to end home isolation or whether to discontinue precautions in a healthcare setting. Roche Elecys Covid-19 IgMIgG Antibody blood test 58 This is the PHE approved Covid-19 venous blood antibody test that we as doctors have been waiting for to provide good quality evidence of your antibody status.
Coronavirus Test Made By Abbott Labs Wins Emergency FDA Approval. The lab-based serology blood test can detect the antibody IgG which identifies if a person has had the novel coronavirus. Heres how to find out.
Abbott Laboratories Inc. This is called the specificity of the test. Abbott is a leader in providing antibody testing at large scale on multiple systems which is helping meet the needs of laboratories as they look to build testing capacity In March Abbot secured FDA EUA status for its fastest available molecular point-of-care test to detect Covid-19 disease.
Visa MC AMEX eCheck Paypal Work time. Food drug and mass merchandiser retailers in the coming weeks for self-use. Rapid Coronavirus Test From Abbott Labs Receives Emergency Approval.
Comparison of the performance of a SARS-CoV-2 Spike-Protein ELISA and the nucleocapsid-based Abbott ArchitectTM SARS-CoV-2 IgG assay indicated that the assays had high concordance with rare paired discordant tests results. The test has been authorized only for the detection of the IgM antibody against SARS-CoV-2 not for any other viruses or pathogens and is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostic tests for detection andor diagnosis of COVID-19 under Section 564b1 of the Act. False positive results can be minimized by choosing an antibody test with high specificity and by testing populations and people who are likely to have had COVID-19.
Last month it launched a real-time polymerase chain-reaction test Abbott m2000 RealTime SARS-CoV-2 test and a rapid point-of-care test for its ID NOW. As of May 14th there are 179 available. The tests help some people decide which precautions to take to curb the spread of Covid-19 but doctors say it isnt known what level of antibodies effectively prevents infection or.
Abbott plans to seek EUA from the US FDA for the test soon. But the validation tests these companies have performed varied widely in size. Purchase the BinaxNOW COVID-19 Antigen Self Test at a retail store near you and perform the test with a simple nasal swab in the comfort and convenience of your home.
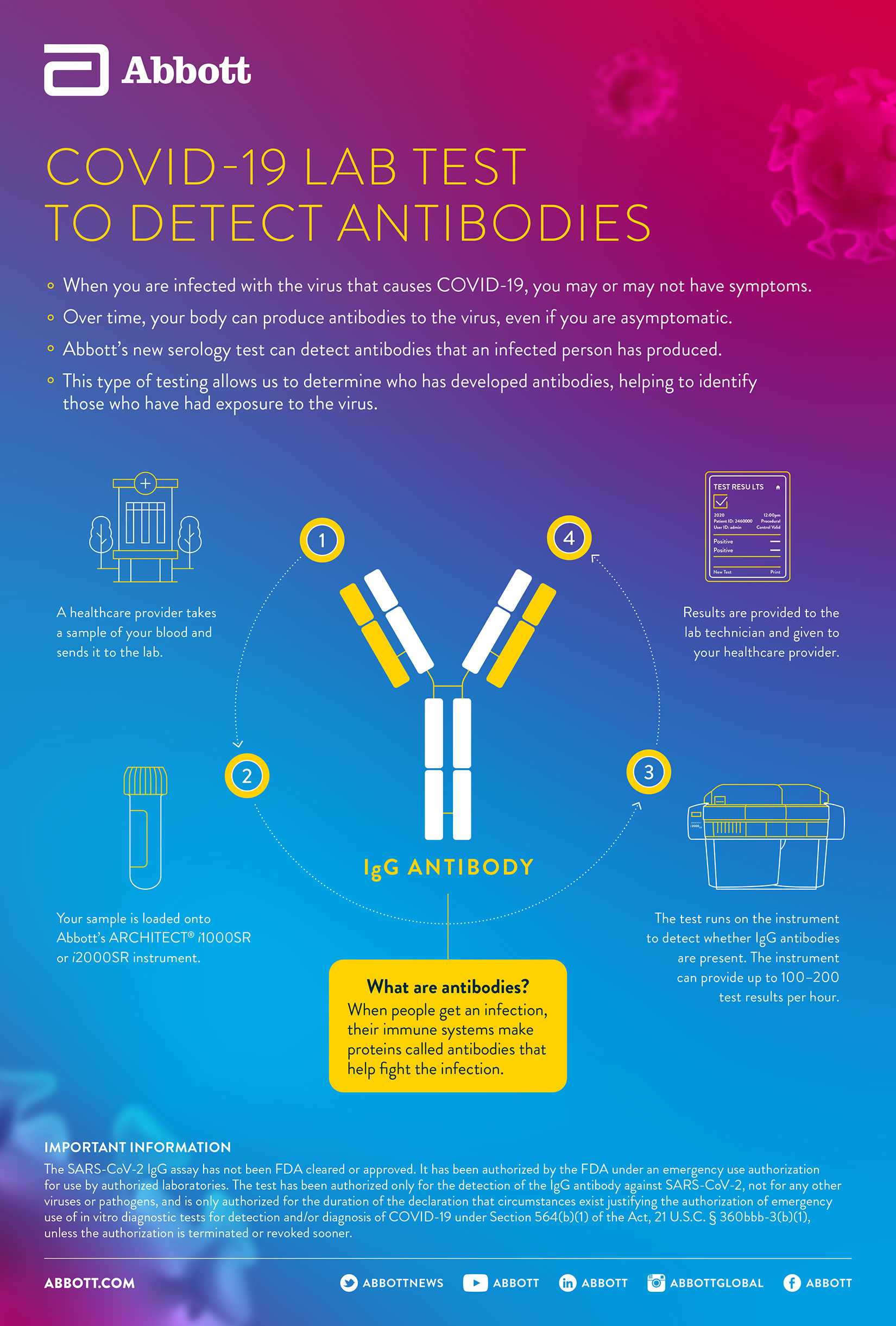
This antibody test adds to Abbotts existing COVID-19 molecular tests that are already being used our m2000 lab test and our rapid ID NOW point-of-care test. Last month Abbott received emergency certification from US. Abbott has received CE Mark for its new quantitative SARS-CoV-2 Immunoglobulin G IgG lab-based serology test that can measure a persons immune response.
Abbotts Covid-19 IgG quantitative antibody blood test obtains CE Mark. 998 Sensitive at detecting IgMIgG antibodies in people who have been ill from Covid-19 have had the vaccine at least 14. Find where Abbotts COVID-19 antibody testing is available in the US.
The very best prices available today fast delivery. Discover the fast proven and trusted COVID-19 antigen test that is readily available over-the-counter at retailers across the country. This coronavirus test tracker displays information on all of the commercially available coronavirus tests.
It will be available at major US. The very best prices available today fast delivery. COVID-19 antibody tests can help identify people who may have been infected with the SARS-CoV-2 virus or have recovered from a COVID-19 infection.
Most coronavirus antibody tests focus on these two antibodies as opposed to IgA which is found mainly in the respiratory and digestive tracts. The Abbott test also tells you that the antibodies the test detected are antibodies to the COVID-19 virus 9963 of the time. Visa MC AMEX eCheck Paypal Work time.
This test has a sensitivity of 100 meaning the test will currently identify COVID-19 IgG antibody if it is present in the blood 100 of the time and a specificity of 998 meaning the test will correctly determine that. Antibody Test Covid 19 Uk Abbott And Save Your money. At this time researchers do not know whether the.
The table above summarises some of the accuracy figures that selected manufacturers claim for their serological Covid-19 tests. Abbott has launched a COVID-19 IgG antibody test with plans to ship 1 million tests this week and 4 million tests in total for April. Similarly Abbotts AdviseDx SARS-CoV-2 IgM antibody test has a 9956 specificity and 95 sensitivity for patients tested 15 days after symptoms started.
Antibody testing for novel coronavirus COVID-19 can help you know whether youve had the virus. The Interim Guidance for COVID-19 Antibody Testing in Clinical and Public Health Settings provides detailed information on how to make the best use of antibody tests. The tests have been authorized only for the detection of proteins from SARS-CoV-2 not for any other viruses or pathogens and are only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostics for detection andor diagnosis of COVID-19 under Section 564b1 of the Federal Food Drug and.
National Cancer Institute Unsplash. Abbotts IgG antibody test will initially be available on its Architect i1000SR and i2000SR laboratory instruments. The BinaxNOW COVID-19 Ag Card is available in the US.
Abbotts SARS-CoV-2 IgG test is the third COVID-19 diagnostic developed by the company. Serologic assays developed for SARS-CoV-2 detect different antibody subtypes and are based on different target antigens.

Abbott Launches Covid 19 Antibody Test Abbott Newsroom

Panbio Covid 19 Igg Igm Rapid Test Point Of Care Abbott

Panbio Covid 19 Igg Igm Rapid Test Point Of Care Abbott

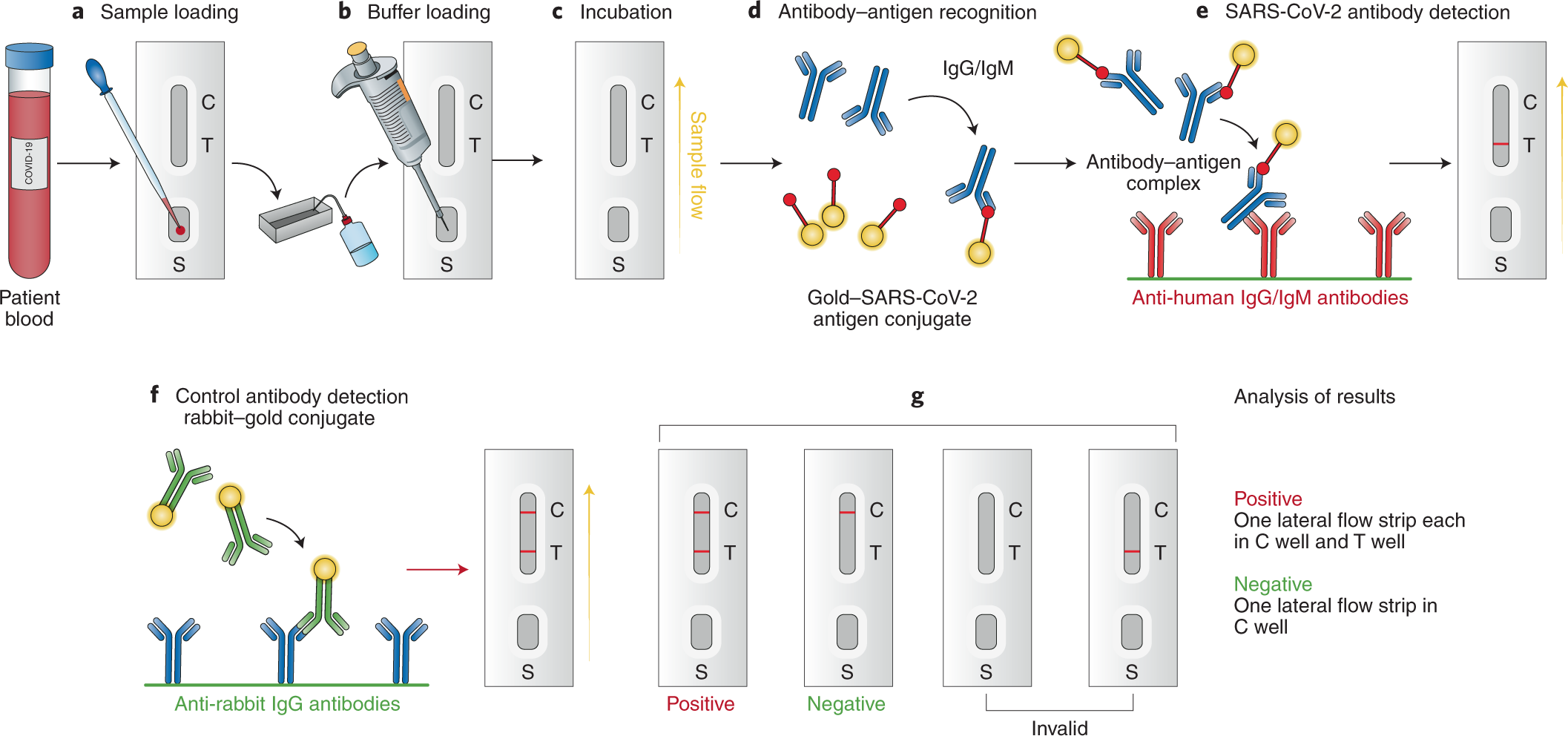
Diagnostics For Sars Cov 2 Infections Nature Materials

Covid 19 Antibody Tests Aren T A Magic Bullet To Escape Lockdown

Tidak ada komentar:
Posting Komentar